“We care for cancer patients by researching on new tools to their doctors”,
Orfeu Flores,
LungCARD coordinator
What is it?
“Blood test for clinical therapy guidance of non-small cell lung cancer patients”Grant Agreement nº: 734790
H2020-MSCA-RISE-2016 - Research and Innovation Staff Exchange
Lung cancer is the most common cancer worldwide. NSCLC alone make up about 75% of all lung cancers and most hospitals currently test all NSCLC patients for EGFR mutations (pharmacogenomics) for treatment decision (personalised medicine) – i.e., patients with mutation(s) in EGFR gene should receive a EGFR-Tyrosine Kinase Inhibitor (TKI) drug (e.g. afitinib) treatment; while those that do not present mutations in such gene, should be treated with chemotherapy. Currently, the laboratories use PCR and Sanger sequencing technologies to perform the EGFR analysis from tumour biopsies - Fixed Paraffin Embedded (FFPE) samples. Still, some patients (e.g., 30% in UK) may never get histological confirmation because they are too sick to make a biopsy. Furthermore, the results obtained with current methods still present low quality, mainly due to poor quality/low yield of DNA extracted from FFPE samples. The FP7 LungCARD project (www.lungcard.eu) has developed and demonstrated a LungCARD system - an automatic system composed by microfluidic chip and chip analyser - that allows to capture circulating tumour cells (CTCs) from blood samples, amplify by multiplex PCR and detect EGFR mutations, including also a software for data analysis and report. Although this new blood test has proven to be faster, cost-effective and human error-free, the detection of somatic mutations in EGFR gene at frequencies lower than 20% is still a weak point. Therefore, the main project’s goal is to benefit from this technology, through the development, improvement, integration and validation of the LungCARD system with NGS workflow and development of a software for automatic reporting clinical results. However, LungCARD project aims to go further, by putting together a global and unique network of multidisciplinary scientists for exchange of knowledge and research training focused on non-small cell lung cancer.
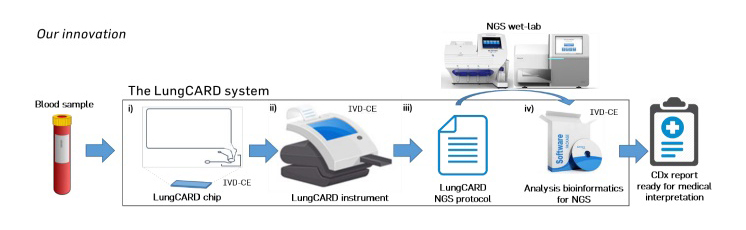
NSCLC patient's blood sample is loaded into the LungCARD chip which is processed in the LungCARD instrument. Afterwards, the outcoming PCR products are then analysed off-chip by NGS following the supplied LungCARD NGS protocol and the results are analysed by the provided bioinformatics software, which automatically delivers report of the companion diagnostic (CDx) ready for medical interpretation.
Who we are?
Consortium worldwide competences, synergies & complementarities
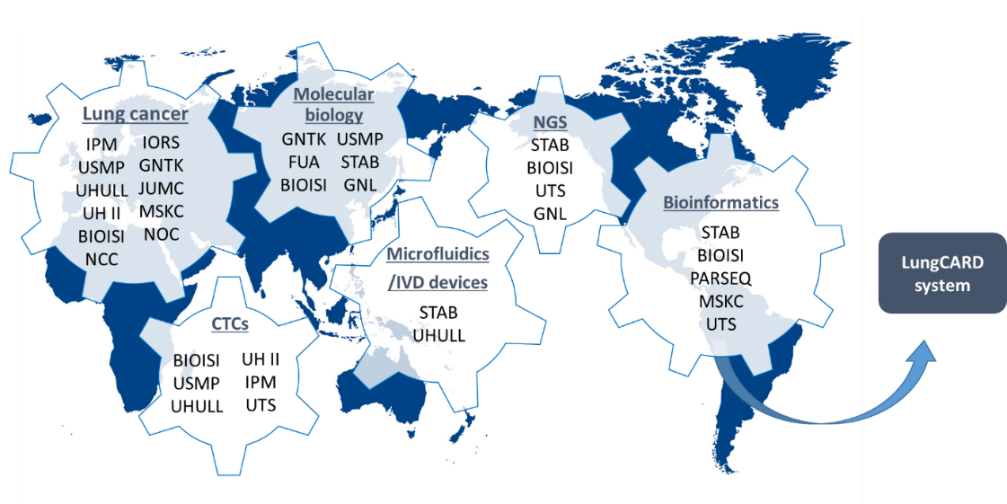
Expertise and contribution of each participant in the Project activities
| Participant | Expertise | Contribution(s) |
|---|---|---|
|
STAB VIDA
(STAB VIDA, Investigação e Serviços em Ciências Biológicas) |
Expert in genetics, IVD, NGS and bioinformatics. Former coordinator of FP7 LungCARD project. | Coordinate LungCARD – RISE (WP1) consortium, dissemination and knowledge transfer (WP7). Gather know-how on different clinical environments (WP2) and FTO (WP3). Develop LungCARD reagents for library prep, NGS workflow, and support development of other reagents and bioinformatics pipelines (WP4). Support validation and implementation of LungCARD system (WP5 & 6). |
|
USMP
(Universidad San Martin de Porres) |
Expert in Human Genetics focused in Peruvian (native and admixed) populations; research and diagnosis of diseases (cancer), immuno- and pharmacogenetics. | Share know-how on Peruvian current practices and regulatory issues (WP2 & 3); Support LungCARD system development (WP4) and local validation (WP5). |
|
UHULL
(University of Hull) |
Expert in Lung cancer pharmacogenomics, as well as microfluidics and IVD devices development. Former partner of FP7 LungCARD project. | Share know-how on UK current practices and regulatory issues (WP2 & 3); Develop LungCARD chips, device and system integration (WP4); Support LungCARD system validation (WP5) and implementation (WP6). |
|
GNL
(Genologica Medica) |
Expert in genetics and clinical pharmacogenomics. | Share know-how on Spanish current practices and regulatory issues and support STAB VIDA in gathering worldwide overview (WP2 & 3); Support LungCARD system development (WP4) and local validation (WP5). |
|
IPM
(Institut Pasteur Du Maroc) |
Expert in oncology and genetics in Morocco. | Share know-how on Moroccan current practices (WP2); Support LungCARD system development (WP4) and local validation (WP5). |
|
UH II
(Universite Hassan II de Casablanca) |
Expert in Moroccan oncogenetics and related molecular biology. | Share know-how on Moroccan current practices and regulatory issues (WP2 & 3); Support LungCARD system development – molecular biology (WP4) and local validation (WP5). |
|
IORS
(Institut Za Onkologiju I Radiologiju Srbije) |
Expert in clinical and medical oncology, and molecular pharmacogenomics. | Share know-how on Serbian current practices and regulatory issues (WP2 & 3); Support LungCARD system development (WP4). |
|
GNTK
(Genetiks Saglik Hizmetleri Tic) |
Expert in genetic diagnosis and molecular biology kit design. | Share know-how on Turkish current practices and regulatory issues (WP2 & 3); Support LungCARD system development (WP4). |
|
PARSEQ
(Parseq Lab) |
Expert in genetic diagnosis bioinformatics | Share know-how on Russian current practices and regulatory issues (WP2 & 3); Support LungCARD bioinformatics development (WP4). |
|
UTS
(University of Technology Sydney) |
Expert in oncogenetics and bioinformatics | Share know-how on Australian current practices and regulatory issues (WP2 & 3); Support LungCARD reagents and bioinformatics development (WP4) and bioinformatics validation (WP5). |
| FUA (Federal University of Agriculture) | Expert in molecular biology | Share know-how on Nigerian current practices and regulatory issues (WP2 & 3); Support LungCARD system development (WP4) |
| NCC (National Cancer Center of Uzbekistan) | Expert in Uzbekistanian oncogenetics and pharmacogenomics | Share know-how on Uzbekistanian current practices and regulatory issues (WP2 & 3); Support LungCARD system development (WP4) and local validation (WP5). |
|
JUMC
(Uniwersytet Jagiellonski) |
Expert in oncology (CTCs and Lung cancer pharmacogenomics) and molecular biology | Share know-how on Polish current practices and regulatory issues (WP2 & 3); Support LungCARD reagents and molecular reactions development (WP4) and local validation (WP5). |
|
MSK
(Sloan-Kettering Institute for Cancer Research corporation) |
Expert in oncogenetics (CTCs and Lung cancer) and bioinformatics | Share know-how on US current practices and regulatory issues (WP2 & 3); Support LungCARD bioinformatics development (WP4) and bioinformatics validation (WP5). |
|
NOC
(Milli Onkologiya Merkezi) |
Expert in Azerbaijani oncogenetics and pharmacogenomics | Share know-how on Azerbaijani current practices and regulatory issues (WP2 & 3); Support LungCARD system development (WP4) and local validation (WP5). |
|
FFCT
(Fundação da Faculdade de Ciências da Universidade de Lisboa) |
Expert in cancer (CTCs) research and bioinformatics | Develop LungCARD reagents for CTC capture and bioinformatics (WP4). Support validation of LungCARD system and bioinformatics (WP5). |
Where we are?
News

Scientists have found that unstable chromosomes within lung tumours increase the risk of cancer returning after surgery, and have used this new knowledge to detect relapse long before standard....

KEYTRUDA is used to treat a kind of lung cancer called non–small cell lung cancer (NSCLC). KEYTRUDA may be used as your first treatment option when your lung cancer has spread (advanced NSCLC) and tests positive for “PD-L1” and your tumor does not have an abnormal “EGFR” or “ALK” gene...

Transgene has started Phase II efficacy testing of its therapeutic cancer vaccine TG4010 and Bristol-Myers Squibb’s checkpoint inhibitor Opdivo (nivolumab) in non-squamous non-small cell lung cancer (NSCLC)...